Genome sequencing is the process of determining the complete DNA sequence of an organism’s genome. The genome is the entire set of genetic material that an organism carries, including all of its genes and non-coding regions.
The first genome sequencing project was the Human Genome Project, which was completed in 2003. Since then, advances in sequencing technology have made it faster, cheaper, and more accurate to sequence genomes.
There are two main approaches to genome sequencing: whole genome sequencing and targeted sequencing. Whole genome sequencing involves sequencing the entire genome, while targeted sequencing focuses on specific regions of the genome that are of interest.
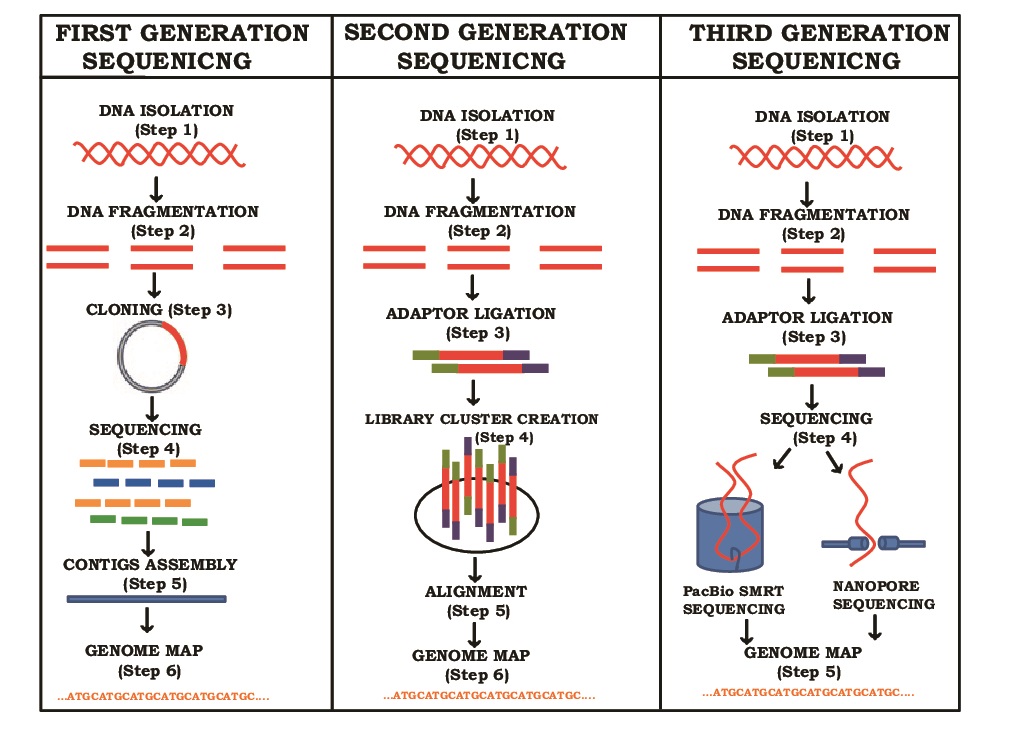
The methodology and working principle of these third-generation sequencings are different from earlier sequencing methods.
The field of microbial genome sequencing has been transformed by advancements in DNA sequencing technologies, particularly with the emergence of next-generation sequencing (NGS). NGS has revolutionized every aspect of microbial genome sequencing. A significant development in this area is the use of multiple displacement amplification reactions (MDA), which can amplify a single microbial genome by a billion-fold. This has made NGS a powerful tool in microbial genomics.
Advanced Commercial Sequencing Platforms
There are multiple commercial platforms available for microbial genome sequencing, including Illumina, Ion Torrent, Roche 454, and Oxford Nanopore. These platforms use different methods to sequence DNA and have differences in their sequencing chemistry, detection method, and molecular numbers during the sequencing process. These platforms can be classified based on these differences.
These sequencing platforms have similarities as well as differences depending on the chemistries and detection methods.

Metagenomics is a field of genomics that involves the direct analysis of DNA from microbial communities without the need for culturing the microorganisms. With the advancement of next-generation sequencing, it is now possible to obtain a complete profile of the entire DNA of a sample through de novo sequencing and metagenomic analysis. This has resulted in significant improvements in genomics analysis.
Genomic and metagenomic assembly processes in which short DNA reads generated by high-throughput sequencing technologies are stitched together to reconstruct the original DNA sequence. The resulting genome or metagenome can then be analyzed to understand the genetic makeup of the organism or community of organisms being studied. There are several software tools available for performing genome and metagenome assembly, each with its strengths and weaknesses including Velvet, MetaVelvet, ABySS, SOAP, SPAdes, Ray Meta, Meta-IDBA, MIRA4, MetaAMOS, and Newbler.
The HapMap has great potential as a valuable tool for advancing our knowledge of the genetic factors that influence health and disease. However, fully realizing its benefits will require collaboration among various fields, including basic science, population genetics, epidemiology, clinical research, social science, ethics, and engagement with the public.
CRISPR–Cas technologies have revolutionized the study of genetics and epigenetics, allowing researchers to investigate gene function and regulatory mechanisms at a deeper level. These technologies have enabled researchers to knock out important genes, modify the epigenetic profile of DNA, and correct mutations responsible for hereditary diseases. The potential of CRISPR-Cas systems in clinical practice has also been promising, with the possibility of developing novel therapeutic approaches for various human diseases. Overall, CRISPR-Cas technologies have opened up new avenues for advancing our understanding of genetics and developing new treatments for genetic disorders.
Nanopore sequencing is a modern approach to DNA sequencing that has quickly evolved to address the need for longer read lengths, faster sequencing, and lower costs. Some publications refer to nanopore sequencing as the fourth generation of DNA sequencing technology.
NGS (Next generation sequencing technology) has also greatly enhanced the study of protein binding sites in genomic DNA, particularly with regards to transcription factor binding sites, using a technique known as Chromatin ImmunoPrecipitation (ChIP). The improved accuracy and sensitivity of NGS technology have enabled the identification and analysis of protein-DNA interactions with greater precision, facilitating a more comprehensive understanding of gene regulation and its impact on cellular processes.
Single-molecule sequencing refers to techniques that can read the base sequence directly from individual strands of DNA or RNA present in a sample of interest.
Long-read sequencing offers a solution to the challenge of accurately sequencing full-length transcripts, allowing for the identification of potentially important isoforms that may have been missed with previous sequencing methods. Recent studies using various types of long-read sequencing, including bulk, single-cell, and targeted sequencing, have shown that many relevant isoforms are still absent from our current transcript annotations. These findings suggest that long-read sequencing can greatly enhance our understanding of gene expression and provide valuable insights into the complexity of transcriptomes.
Complex diseases are illnesses that are influenced by multiple genes or variants, making it difficult to apply the same methods used for Mendelian diseases. Unlike Mendelian diseases, variants that contribute to complex disease susceptibility tend to have a moderate effect size. To study complex diseases, researchers use genome-wide association studies (GWAS). GWAS is designed to identify genetic variations associated with complex diseases by examining the entire genome rather than focusing on individual genes.
Applications
Genome sequencing has a wide range of applications in various fields including bioinformatics. Here are some applications of genome sequencing:
- Personalized Medicine: Genome sequencing can provide insights into an individual’s genetic makeup, which can help doctors develop personalized treatment plans.
- Disease Diagnosis: It can diagnose diseases by identifying genetic variations that are associated with certain illnesses.
- Drug Development: Also can aid in the development of new drugs by identifying potential drug targets and understanding how drugs interact with genes.
- Agriculture: Here, it can be used to improve crop yields and develop disease-resistant crops.
- Forensics: Helps identify individuals in forensic investigations.
In the field of bioinformatics, genome sequencing can be used for various purposes, such as:
- Sequence Analysis: This could provide large amounts of data that can be analyzed to identify genetic variations and study gene expression patterns.
- Comparative Genomics: Useful for the comparison of genetic makeup of different organisms, providing insights into the evolution and species relationships.
- Functional Genomics: Assists researchers understand how genes function and how they interact with each other.
- Systems Biology: Genome sequencing can be used to study the complex interactions between genes, proteins, and other molecules in biological systems.
Conclusion
In conclusion, genome sequencing has undergone tremendous advancements over the years, with the development of second-generation sequencing and more recently, third-generation sequencing technologies. These advancements have led to the generation of vast amounts of genomic data and have opened up numerous possibilities for applications in various fields. The ability to sequence genomes has enabled researchers to study the genetic basis of diseases, identify new drug targets, improve crop yield, and better understand the evolutionary history of species. With continued technological advancements and decreasing costs, genome sequencing is poised to continue making significant contributions to various fields, leading to new discoveries and improving our understanding of the world around us.
References
- Artificial intelligence (AI) and big data in cancer and precision oncology, “August 2020 Computational and Structural Biotechnology Journal 18″,(4) DOI:10.1016/j.csbj.2020.08.019
- Srivastav R, Suneja G. Recent Advances in Microbial Genome Sequencing (Microbial Genomics in Sustainable Agroecosystems.). Singapore: Springer;2019
- Lasken RS (2007), “Single-cell genomic sequencing using multiple displacement amplification”, Curr Opin Microbiol 10(5):510–516
- Ashkenasy N, Sánchez-Quesada J, Bayley H, Ghadiri MR (2005) Recognizing a Single Base in an individual DNA Strand: a step toward DNA sequencing in Nanopores. Angew Chem Int Ed
44(9):1401–1404
- Handelsman J (2004),” Metagenomics: application of genomics to uncultured microorganisms”, Microbiol Mol Biol Rev 68(4):669–685
- Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using de Bruijngraphs. Genome Res 18:821–829.
- “The International HapMap Project”, The International HapMap Consortium, Nature volume 426, pages789–796 (2003)
- “Recent Advances in Genome-Engineering Strategies”, Michaela A. Boti et.al;, Genes 2023, 14(1), 129;
- “DNA sequencing: an overview of solid-state and biological nanopore-based methods”, Mohammad M. Mohammadi, https://doi.org/10.1007/s12551-021-00857-y, Biophysical Reviews (2022) 14:99–110.
- Lin B, Hui J, Mao H (2021) Nanopore technology and its applications in gene sequencing. Biosensors 11:214. https://doi.org/ 10.3390/bios11070214.
- “Next generation sequencing technology: Advances and applications”, H.P.J. Buermans, Volume 1842, Issue 10, October 2014, Pages 1932-1941,
- Single-Molecule Sequencing. In: Roberts, G.C.K. (eds) Encyclopedia of Biophysics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-16712-6_498.
- Au KF, Sebastiano V, Afshar PT, Durruthy JD, Lee L, Williams BA, et al.Characterization of the human ESC transcriptome by hybrid sequencing. Proc Natl Acad Sci. 2013; 110(50):4821–30. https://doi.org/10.1073/pnas.1320101110.
- Anders, L. et al. Genome-wide localization of small molecules. Nat. Biotechnol. 32, 92–96 (2014).
- Cardon, L. R., and Bell, J. I. (2001) Association study design for complex diseases. Nat. Rev. Genet., 2, 91–99