Of the many advancements made in molecular biology, the most valuable is the PCR. PCR has extensive applications in a wide range of fields in molecular biology and biotechnology, including basic and applied research, DNA analysis and manipulation, constructing transgenic organisms, forensic science, medical diagnosis, gene therapy, and environmental analysis. PCR has also been adapted to measure the levels of mRNA in various tissues or organisms, providing insights into the cellular environment and the expression of genes that help the organism adapt to changes.
Conventional PCR: Amplifying Target DNA Sequences for Various Applications
This is a standard PCR process where the primers bind with DNA strands. Here the primers bind specifically to each other with 2 DNA strands. The primers used in PCR are specific to the target sequence, limiting the replication to that particular DNA sequence. The process can be performed in PCR tubes containing aluminum blocks, DNA polymerase, buffer, target DNA, and primers. It takes about 35-40 minutes to complete and is monitored using gel electrophoresis. PCR can be conducted using Prime STAR HS kits. The PCR reaction mixture usually contains 50 microliters, with 10 microlitres of genomic DNA content.
The result of conventional PCR are the identical copies of the target DNA sequence used for a variety of applications, including genetic research, medical diagnosis, and forensic analysis.
Multiplex PCR for Simultaneous Detection of Multiple Pathogens and Gene Sequences
Multiplex PCR is a technique that detects multiple pathogens in a single sample and can identify exonic/intronic sequences in specific genes. The primers designed for this technique are specific to a DNA sequence, with different base pair lengths to create distinct bands. This method saves time and expense by targeting varying sizes of different DNA genes in a single reaction and can detect viral, bacterial, and other infectious agents. However, using more than one primer pair can increase the risk of primer-dimer amplification and discrimination of DNA layer fragments. Multiplex PCR uses Taq polymerase additive to decrease competition among amplicons. Examples of this technique include Brucella diagnostics based on the perosamine synthetase gene, the identification of major species of genes such as bcsp 31, omp 2b, omp2a, omp 31, and a 19-primer multiplex PCR for the identification of B. neotomae, B. ceti, and B. microti.
Real-Time PCR in Molecular Diagnostics
Real-time PCR technology has made a significant impact on molecular diagnostics in recent years. Several vendors offer different systems that have a small footprint and are relatively affordable. Quantitative PCR (qPCR) combines DNA amplification with measuring the concentration of a specific DNA sequence in the reaction. This enables the calculation of the initial template concentration and is commonly used for analyzing DNA copy number, viral load, SNP detection, and allelic discrimination. Additionally, qPCR can be used to measure mRNA expression when coupled with reverse-transcription PCR and is regarded as the standard method for validating microarray gene expression data.
A good example of how real-time PCR can is the analysis of cerebrospinal fluid specimens for herpes simplex virus infection. Real-time PCR is a technique that combines amplification and detection in one step, allowing the detection of the amplicon as it is produced in real-time. This is done by adding a fluorescent signal to the amplicon and detecting it using a specialized thermal cycler. Fluorescent signals are generated during amplification using either a dye like SYBR Green or an oligonucleotide labeled with a fluorogenic dye that is used as a primer or probe. While only a limited number of real-time PCR tests are currently approved by the FDA, many more are performed daily in molecular pathology labs using laboratory-developed assays and analyte-specific reagents.
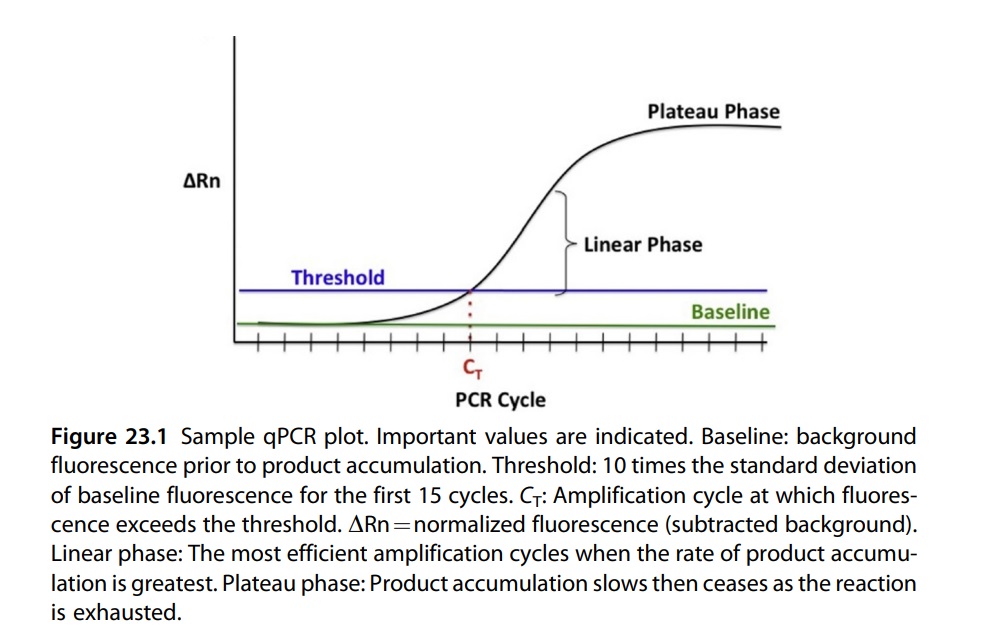
Real-time PCR and conventional PCR differ in various ways. Real-time PCR usually eliminates post-amplification processing, such as gel electrophoresis, which is required in conventional PCR. In conventional PCR, the amplicon needs to be assessed in post-amplification analysis. This poses a risk of contamination and false-positive results even with minimal amounts of the amplicon. Eventually, conventional PCR will be phased out and replaced by real-time PCR, as more laboratories switch to real-time technologies.
Reverse Transcriptase PCR (RT-PCR): Amplifying RNA for Diagnosis and Quantification
Also known as quantitative reverse transcriptase PCR (qRT-PCR), this technique is used to amplify RNA sequences by first converting them into complementary DNA (cDNA) using the reverse transcriptase enzyme. RT-PCR was made possible due to the discovery of retroviral reverse transcriptase, which is an RNA-dependent DNA polymerase that catalyzes DNA synthesis using RNA as a template. The synthesized DNA is known as complementary DNA (cDNA) which is more stable than RNA and is not susceptible to degradation by RNase. In RT-PCR, RNA is first extracted, reverse transcribed into cDNA, and subsequently degraded. Amplification of dsDNA then occurs using PCR. RNA purification kits for both manual and automated RNA extraction are available, which make RNA analysis in the clinical laboratory as quick and sensitive as PCR-based DNA amplification. RT-PCR is commonly used to diagnose and quantify RNA virus infections such as HIV and HCV, and to analyze mRNA transcripts associated with non-Hodgkin’s lymphomas, leukemias, and sarcomas. Gene expression profiling, which is expected to have a significant impact on molecular diagnostics in the future, will likely rely on RNA analysis using RT-PCR.
Nested PCR: Enhancing PCR Sensitivity and Specificity
Nested polymerase chain reaction (PCR) is applied when there is a need to boost the sensitivity and/or specificity of PCR, for instance, when amplifying a specific member of a polymorphic gene family or when amplifying a cDNA copy of an mRNA found in a clinical specimen containing a diverse population of cell types and with low abundance. This method consists of two rounds of PCR amplification, with the first round using outer primers that amplify a larger region of DNA, and the second round using nested primers that amplify a smaller, more specific region within the first PCR product. The use of two pairs of oligonucleotides permits an increased number of cycles to be performed, thereby heightening the sensitivity of the PCR. The enhanced specificity of the reaction is due to the binding of two distinct sets of primers to the same target template. Nested PCR is an effective approach for amplifying long template segments, but it requires familiarity with the target sequence. Nested PCR can elevate the sensitivity and specificity of PCR, which makes it valuable for identifying low-abundance DNA sequences, genetic mutations, and pathogen detection.
Multiplex PCR: Detecting Multiple DNA Targets in a Single Reaction
Multiplex PCR involves amplifying multiple target DNA sequences in a single reaction. This process uses multiple primers and a temperature-regulated DNA polymerase in a thermal cycler to amplify DNA in samples. Multiplex PCR can save time, reduce sample input, and increase throughput by detecting multiple DNA targets in a single reaction. It is commonly used in pathogen detection, genotyping, and forensic science. It was initially introduced in 1988 as a means to detect deletions in the dystrophin gene and later utilized for the steroid sulfatase gene. In 2008, multiplex-PCR was employed for the analysis of microsatellites and Single Nucleotide Polymorphism (SNP). This technique involves the use of multiple sets of primers in a single PCR mixture to generate amplicons of different sizes, which are specific to various DNA sequences. By targeting multiple sequences simultaneously, additional information may be obtained from a single test run that would otherwise require a larger quantity of reagents.
Digital PCR: Revolutionizing the Precise Quantification of DNA in Molecular Biology Research
Digital PCR (dPCR) is a novel technique that enables precise quantification of nucleic acids by using a limiting dilution analysis combined with Poisson distribution analysis. This technique partitions the DNA sample into thousands of tiny droplets, each containing a single DNA molecule. The amplification reaction system used in dPCR is similar to that of standard PCR. A droplet generator is used to split each dPCR reaction mixture into 20,000 nL droplets, with each droplet containing either zero or one or more copies of the target DNA. After a standard PCR procedure, the number of positive and negative droplets is counted and recorded by a drop reader. Finally, the Poisson distribution law is used to calculate the precise number of genomic target measurements in copies per microliter on one chip in the samples. The use of a nanofluidic chip allows for the rapid and easy execution of thousands of parallel PCR reactions. dPCR has the potential to accurately and sensitively measure the number of copies of target DNA, particularly for samples with low concentrations and complex mixtures. Digital PCR is used in rare allele detection, copy number variation analysis, and mutation detection.

Advantages and Limitations of Different PCR Techniques in Molecular Biology Research
In conclusion, PCR techniques have revolutionized molecular biology research by enabling the amplification and quantification of DNA sequences. The different types of PCR, including conventional PCR, qPCR, nested PCR, multiplex PCR, and digital PCR, have unique features and advantages that make them suitable for specific applications. Conventional PCR is simple, inexpensive, and efficient, while qPCR provides quantitative information and is highly sensitive. Nested PCR and multiplex PCR increase the specificity and sensitivity of PCR and allow for the detection of multiple targets simultaneously. Digital PCR enables absolute quantification of DNA targets and provides greater precision for low-abundance targets. Each PCR technique has its strengths and limitations, and researchers must choose the appropriate method for their specific research needs.
References
- Clark, D. P., Pazdernik, N. J., & McGehee, M. R. (2019). Polymerase Chain Reaction. Molecular Biology, 168–198. doi:10.1016/b978-0-12-813288-3.00006-9
- https://faculty.ksu.edu.sa/sites/default/files/different_types_of_pcr_techniques_and_its_applications.pdf
- Dymond, Jessica S. (2013). [Methods in Enzymology] Laboratory Methods in Enzymology: DNA Volume 529 || Explanatory Chapter. , (), 279–289. doi:10.1016/B978-0-12-418687-3.00023-9
- Farkas, Daniel H. (2009). Cell and Tissue Based Molecular Pathology || Overview of Molecular Diagnostic Techniques and Instrumentation.
- Green MR, Sambrook J. Nested Polymerase Chain Reaction (PCR). Cold Spring HarbProtoc. 2019 Feb 1;2019(2). doi: 10.1101/pdb.prot095182. PMID: 30710024.
- Nazir, R., Rehman, S., Nisa, M., & Baba, U. ali. (2019). Exploring bacterial diversity. Freshwater Microbiology, 263–306. doi:10.1016/b978-0-12-817495-1.00007-4
- Espiñeira, M., & Lago, F. (2016). Advances in Authenticity Testing for Fish Speciation. Advances in Food Authenticity Testing, 415–440. doi:10.1016/b978-0-08-100220-9.00015-1